RESEARCH
Our
research focuses on understanding and controlling the effects of additives
on the physicochemical behavior of biological and synthetic macromolecules,
micelles and nanoparticles in general (see figure below) in aqueous
mixtures.

Our
research is currently focusing on two specific phenomena here denoted as
macromolecular condensation and particle diffusiophoresis. They are
described below.
Macromolecular Condensation
Macromolecular
condensation refers to the formation of macromolecule-rich spherical liquid
microdroplets inside aqueous liquid media. It is a liquid-liquid phase separation (LLPS) transition, which has attracted
great attention in the case of proteins. Protein
condensation is
typically observed by cooling protein aqueous solutions. Next figure
illustrates the formation of a protein-rich droplet from cooling a
protein-water solution. A picture with actual protein-rich micro-droplets
is also included.
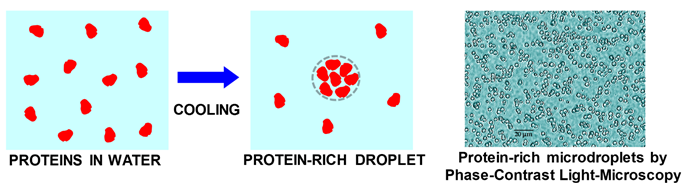
Protein
condensation is a reversible process as expected for phase transitions.
Next picture shows the opacification of a protein aqueous sample induced by
cooling. Sample transparency is restored by heating. Click the link below
the picture to watch the reversible opacification of a protein sample.
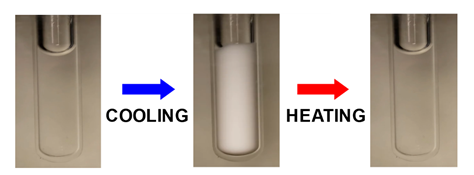
Click: Reversible opacification of a
protein sample
Protein
condensation is known to drive the formation of membraneless organelles
inside living cells and it is believed to be an important intermediate for
the formation of pathological protein aggregates. Protein-rich
microdroplets are also known to be metastable with respect to protein
crystallization and can become sites for the nucleation of protein
crystals. Understanding and controlling the formation of protein droplets
and their transformation into protein crystals or aggregates is important
for
·
Protein crystallization, which is important for the
characterization of protein three-dimensional structure by traditional
X-ray crystallography or emerging crystallographic methods such as X-ray
free-electron laser and micro-crystal electron diffraction techniques.
·
Preparation of protein-based materials with applications in
catalysis, drug delivery and sensing.
·
Inhibition of protein aggregation in pharmaceutical
formulations containing therapeutic proteins such as monoclonal antibodies.
·
Comprehending the role of protein condensation in biological
processes and diseases associated with protein aggregation.
We
have being interested in answering the following
questions:
Can additives induce protein condensation?
Protein
condensation is driven by protein-protein attractive interactions (e.g.,
electrostatic, van der Waals, hydrophobic) in water. It was thought to be a
not-very-common phenomenon, occurring only for few proteins (e.g., eye-lens
crystallins). It is believed that protein condensation in water is not
observed for many protein cases because it would occur well below the
freezing point of water. In other words, protein-protein attractive
interactions are not sufficiently strong. Recently, protein condensation
has been reported for many protein cases. One reason is related to the
continuous production and characterization of many new recombinant proteins
such as monoclonal antibodies. A second important reason can be linked to
the comprehension of another phenomenon of macromolecular aqueous mixtures,
known as macromolecular crowding. This is an alteration
of the state of protein aqueous solutions created by the addition of
“inert” synthetic or natural macromolecular additives. Macromolecular
crowding introduces steric (excluded volume) interactions that increase protein-protein
attractive interactions thereby favoring protein condensation. To
appreciate this phenomenon, we consider an example of people crowding
inside a room. The following figure shows a room with four tables (red
rectangles) and eight persons (blue circles). The persons in the room will
push tables together in order to maximize the space available to them.
People and tables represent the crowding additive and the proteins,
respectively. Joining tables
together represents protein condensation due to macromolecular crowding.
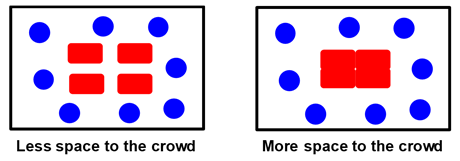
In
one of our previous work (J.
Phys. Chem. B 2007, p1222), we showed that polyethylene glycol (PEG) induces condensation of serum
albumin by macromolecular crowding.
How can we use additives to control the fate of metastable
protein-rich droplets?
In
our research group, we are currently preparing aqueous mixtures in which
protein condensation occurs and investigating how additives such as salts
or organic molecules can control the fate of protein microdroplets (Int. J. Biol. Macromol. 2021, p519 and J. Mol. Liq. 2024, p124164). Next picture
shows the opacification of a protein aqueous sample induced by cooling due
protein condensation. Upon heating, sample transparency is not recovered
because protein-rich microdroplets act as nucleation sites for protein
crystallization.
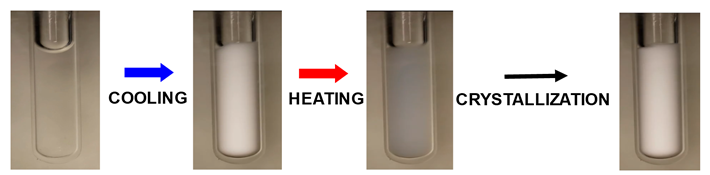
Click: Protein Condensation as a
platform for protein crystallization
Can we observe condensation of other globular macromolecules?
We
are also interested in another type of macromolecules known as dendrimers. These are branched tree-like synthetic polymers with a
globular shape in solution, similar to that of proteins. Dendrimers have being investigated for their ability to host small
molecules with potential applications as drug carriers, solubilizing and
extracting agents and catalyst supports. We inquired whether dendrimer
condensation occurs as in the case of proteins. In a previous work, we have
shown that aqueous solutions of polyamidoamine (PAMAM) dendrimers with
hydroxyl and amino surface functionalities undergo dendrimer condensation
in the presence of sodium sulfate, a strong salting-out agent. To our
knowledge, this represents the first experimental report on dendrimer LLPS.
Not only dendrimer condensation occurs but it also exhibits an unusual
thermal behavior: it can switch from being induced by cooling to being
induced by heating as salt concentration increases (PCCP 2015, p28818).

How is proximity to condensation
conditions influencing aggregation processes?
A
protein solution that is physically stable may be still susceptible to
irreversible chemical processes such as dimerization, oligomerization and
aggregation in general. These processes are involved in the formation of
pathological aggregates. We are
interested in understanding how proximity to LLPS conditions influences aggregation. In one of our previous work, we
mimicked protein oligomerization by adding small amounts of chemical
crosslinkers. In the proximity of LLPS conditions, it was demonstrated that
oligomerization raised LLPS temperature resulting in the rapid formation of
spherical aggregates. In the absence of LLPS, aggregation was slow and
results in the formation of branched aggregates (Langmuir 2008, p2799). The same approach
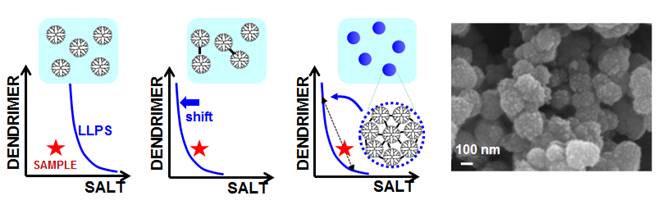
was also extend to dendrimers. As illustrated in
the figure below, a stable dendrimer-salt aqueous sample is prepared with a
composition near the LLPS boundary. A small amount of chemical crosslinking
produces dendrimer dimers. This induces a shift in the LLPS boundary, which
crosses sample coordinate, leading to sample LLPS. Dendrimer-rich droplets
then produce crosslinked dendrimer nanospheres (Langmuir, 2017, p5482). We believe that
oligomerization-induced LLPS may be valuable for the preparation of
nanomaterials with large host capacity from building blocks such as small
dendrimers.
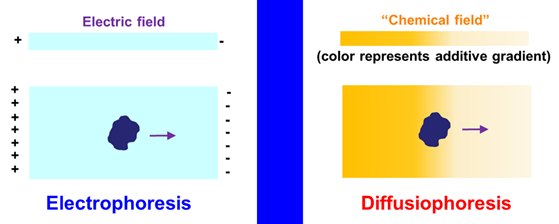
Particle Diffusiophoresis
Diffusiophoresis rhymes
with electrophoresis. We know that electrophoresis is the migration of a
charged particle induced by a gradient of electric potential (electric
field). This particle can be a protein, nucleic acid, a synthetic
polyelectrolyte or a nanoparticle. Diffusiophoresis is instead the
migration of a particle induced by a gradient of chemical potential of an
additive such as a salt. This “chemical field” is established by imposing a
gradient of additive concentration (see review: Molecules,
2024, p1367)
Diffusiophoresis
has attracted much attention as a means to control particle motion in
liquids. Specifically additive gradients could be used to achieve particle
focusing and self-assembly, separation of different macromolecules,
diffusion-based controlled release, enhancement or retardation of particle
adsorption on surfaces and particle insertion into porous materials. Thus,
diffusiophoresis could be exploited in applications such as
·
Protein separation and detection in microfluidics.
·
Enhance protein adsorption onto sensing surfaces (e.g.,
surface plasmon resonance).
·
Use of micelles and other nanoparticles in enhanced oil
recovery and soil remediation.
·
Prevention of membrane fouling in reverse osmosis, forward
osmosis and protein ultrafiltration
Diffusiophoresis has
been observed for model colloidal particles such as polystyrene or silica
particles in the presence of salt gradients. In these cases, this transport
phenomenon can be explained by considering an elecrokinetic mechanism that requires
particles to be electrically charged.
We
have being interested in answering the following
questions:
Does salt-induced
diffusiophoresis occur for neutral particles?
Salt-induced
diffusiophoresis can be driven by particle preference for water (preferential hydration). In other words, a hydrophilic particle may migrate along a
salt gradient in order to reach the region with lower salt concentration
and correspondingly higher water concentration. An important example of
hydrophilic particles is represented by PEG polymer chains.
Understanding PEG diffusiophoresis is also important for controlling the
motion of PEG-based particles, such as micelles, vesicles, PEGylated
proteins and PEG-coated nanoparticles.
In one of our previous work (Langmuir 2014, p4916), we used
precision Rayleigh interferometry (see figure below) to show that a KCl
concentration gradient induces PEG diffusiophoresis from high to low salt
concentration.
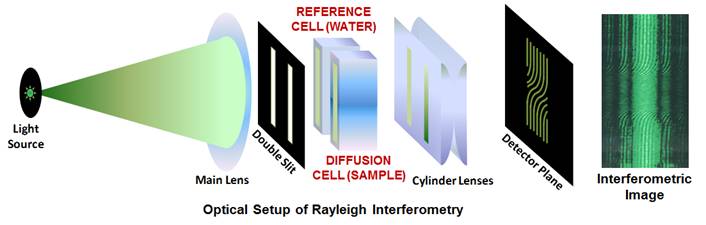
Our lab houses a unique
instrument known as the “Gosting Diffusiometer”. This interferometer
has reported the most precise diffusion coefficients of liquid mixtures.
The Gosting diffusiometer was built by Prof. Louis J. Gosting and then
enhanced and adapted for Rayleigh Interferometry by Dr. Donald G. Miller
and Prof. John G. Albright. We also measure diffusion coefficients by
dynamic light scattering (DLS).
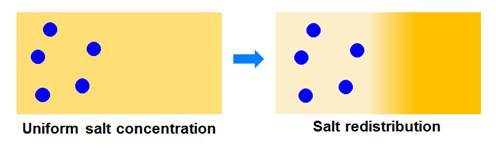
Can we invert the
roles?
A salt concentration
gradient can induce diffusiophoresis of particles. However, a concentration
gradient of particles can also induce motion of salt ions (see figure
below). We denoted this mechanism as salt osmotic diffusion. We have
experimentally characterized this mechanism and showed that it is
theoretically linked to particle diffusiophoresis. Knowledge of salt
osmotic diffusion allows us to identify the thermodynamic and hydrodynamic
components of particle diffusiophoresis (see Langmuir 2015, p12210 for example).
Does salt-induced PEG
diffusiophoresis follow the Hofmeister Series?
In 1888, Franz
Hofmeister first reported that salt ions can be ranked according to their
effectiveness in precipitating proteins from aqueous solutions. This
ranking is known as the Hofmeister series. The overall orders of the anions and
cations are shown in the figure below. Ions on the left side tend to reduce
protein solubility in water (salting-out) while ions on the right side tend
to increase protein solubility (salting-in).
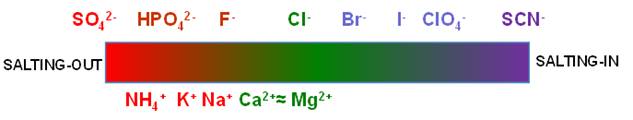
Nowadays, the
Hofmeister series is investigated in many other processes distinct from
protein solubility. it has also been shown to be valid for synthetic
macromolecules. In one of our previous work, we
showed that PEG diffusiophoresis follows the Hofmeister series.
Furthermore, in salting-in conditions, ion binding gives rise to an
electrical charge on PEG chains (Langmuir 2015, p1353).
Do osmolyte gradients
cause diffusiophoresis?
Osmolytes are neutral organic molecules with low molecular weight that
are soluble in water. Some osmolytes are shown in the figure below. Their
name originated from their ability to influence solution osmotic pressure.
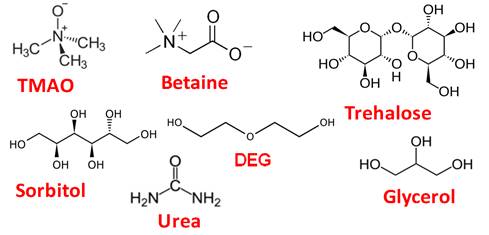
We showed that PEG
diffusiophoresis occurs in the presence of TMAO and DEG from high to low
osmolyte concentration while it is negligible in the presence of urea.
Osmolyte osmotic diffusion was also characterize. PEG diffusiophoresis in
the presence of TMAO is comparable to that observed in the presence of a
strong salting-out agent (Langmuir 2018, p9525).
Does salt-induced
diffusiophoresis occur for PEG-based nanoparticles?
We have characterized
salt-induced diffusiophoresis of PEG chains. However, PEG also governs the
interfacial properties of PEG-based nanoparticles. Our recent work has
focused on the experimental characterization of diffusiophoresis of
PEG-based micelles (J. Mol. Liq. 2022, p119271 and Int. J. Mol. Sci. 2022, p13710). Our
experimental results show that diffusiophoresis of PEG-based micelles in
the presence of Na2SO4 can be approximately linked to
that of PEG chains.
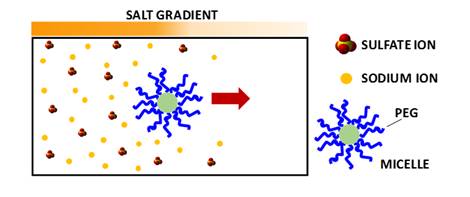
Does salt-induced
diffusiophoresis occur for proteins?
Previous
diffusiophoresis studies have demonstrated salt-induced diffusiophoresis
for charged colloidal particles. Can this phenomenon occur also for charged
proteins? We have reported the first experimental study on protein
diffusiophoresis (J. Phys. Chem. B 2012, p12694). Specifically,
we have characterized salt-induced diffusiophoresis of the protein lysozyme
in the presence of NaCl. Salt osmotic diffusion was also characterized and
can be linked to a thermodynamic phenomenon known as Donnan equilibrium. Experimental results show that salt-induced protein
diffusiophoresis is governed by the electrokinetic mechanism at low salt
concentration and the preferential-hydration mechanism at high salt
concentration. Amplification of salt-induced diffusiophresis of protein is
achieved in the presence of MgCl2, due to cation binding to
protein and salt large osmolarity (Langmuir 2020, p2635).
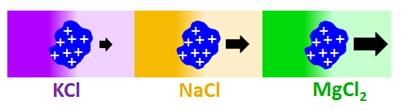
We have also
theoretically examined protein diffusiophoresis, salt osmotic diffusion and
the DLS protein diffusion coefficient in the context of multicomponent
diffusion and the fundamental laws od non-equilibrium thermodynamics (Int. J. Heat Mass Transfer 2020, p120436
and Int. J. Heat Mass Transfer 2023, p124503).
|